Corden Pharma Chenôve
is laureates of France Relance
The project aims to set up new production capacities for very high purity lipids intended for LNPs (Lipid NanoParticles) necessary for the formulation of mRNAs for anti-COVID-19 vaccines.
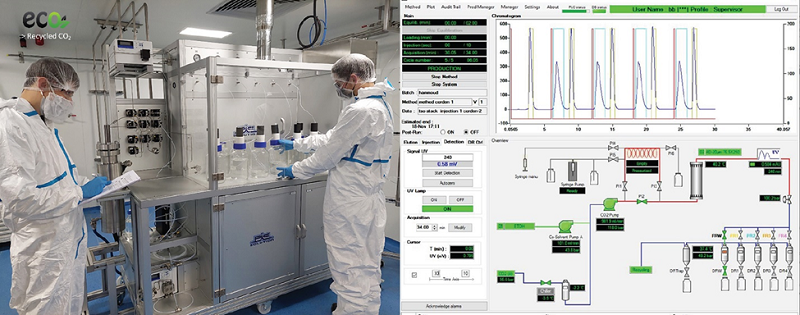
CordenPharma invests in an expansion of its specialty lipids production at CordenPharma Chenôve (FR) using Supercritical Fluid Chromatography (SFC) technology for compound separation, which is an efficient and cost-effective process for purifying lipids and pharmaceutical drug substances.
Because SFC is an eco-friendly and sustainable technique using reclaimed CO2 coupled with online carbon dioxide recycling, the resulting increase in lipid production will be a greener approach to manufacturing highly pure complex lipids, which are essential for not only mRNA-based COVID-19 vaccines, but also for newly developed cell and gene therapies, SiRNA and miRNA programs. Commercial lipid quantities using the new technology will be produced at CordenPharma Chenôve as early as the first half of 2022.
Over the past month, Corden PHARMA has announced several strategic investments:
CordenPharma to Invest €9.7 Million in Highly Potent Oral Solid Drug Product Development & Manufacturing at CordenPharma Plankstadt From early-stage development to full commercial scale, CordenPharma Plankstadt is a Centre of Excellence for the Development & Manufacturing of Highly Potent Oral Solid Drug Products for compounds that have an OEL level < 1 µg/m3.
The installment of the new CTD facility at CordenPharma Plankstadt is a response to Corden Pharma customers' demands and the recognition of gaps in scale, formulation development technologies for insoluble APIs, and manufacturing of capsule products. Joining three other CTD facilities (CTD 1: 1-5 kg GMP; CTD 2: 5-20 kg GMP; CTD 3: non-GMP), CTD 4 will bridge the scale gap between CTD 2 and the two Production Facilities (PF 1 and PF 2) to manufacture GMP batches up to 50 kg. CTD 4 will be equipped with all of the key oral solid dosage manufacturing needs including blending, granulation (high shear, fluid bed, roller compaction), compression, and coating. Investments in high potency filling equipment (powders, pellets, mini-tablets) into capsules, hot melt extrusion (HME), and the ability to operate with organic solvents are also being added. The facility will be designed with multi-product capability, allowing multiple projects to run in parallel. Work has recently started in the fourth quarter of 2021, with a targeted completion date in the third quarter of 2022.

Strategic Investment in Small Molecule API Development At the very beginning of November, Corden Pharma announced the completion of a Phase 1 strategic investment to create a Flow Chemistry Centre of Excellence at its CordenPharma Chenôve facility, near Dijon, France.
Since Flow Chemistry & Continuous Manufacturing play an increasingly significant role in today's pharmaceutical development landscape, CordenPharma Chenôve has, over the last five years, engaged in projects on behalf of customers to address their specific process concerns by employing flow chemistry and continuous manufacturing. Considering these past successes, CordenPharma made the decision to invest in their Small Molecules platform by expanding CordenPharma Chenôve's capabilities in this area by creating and staffing a Flow Chemistry Centre of Excellence.
RELATED 7 CDMOs are laureates of France Relance
